- Articolo
- Fonte: Campus Sanofi
- 22 feb 2024
Fisiopatologia Prurigo Nodularis

Nell’insorgenza delle lesioni della PN sono coinvolte diverse cellule immunitarie, tra cui le cellule dell'infiammazione di tipo 21-8:

Le lesioni da PN sono caratterizzate da un' aumentata presenza e attività delle citochine di tipo 2 IL-4, IL-13 e IL-31, nonché di altri mediatori infiammatori (IL-17, IL-22, pruritogeni e neuropeptidi)4,5

Nella PN, la relazione tra la cute, il sistema immunitario e il sistema nervoso è disregolata3
L'esatta patogenesi della PN rimane sconosciuta2.
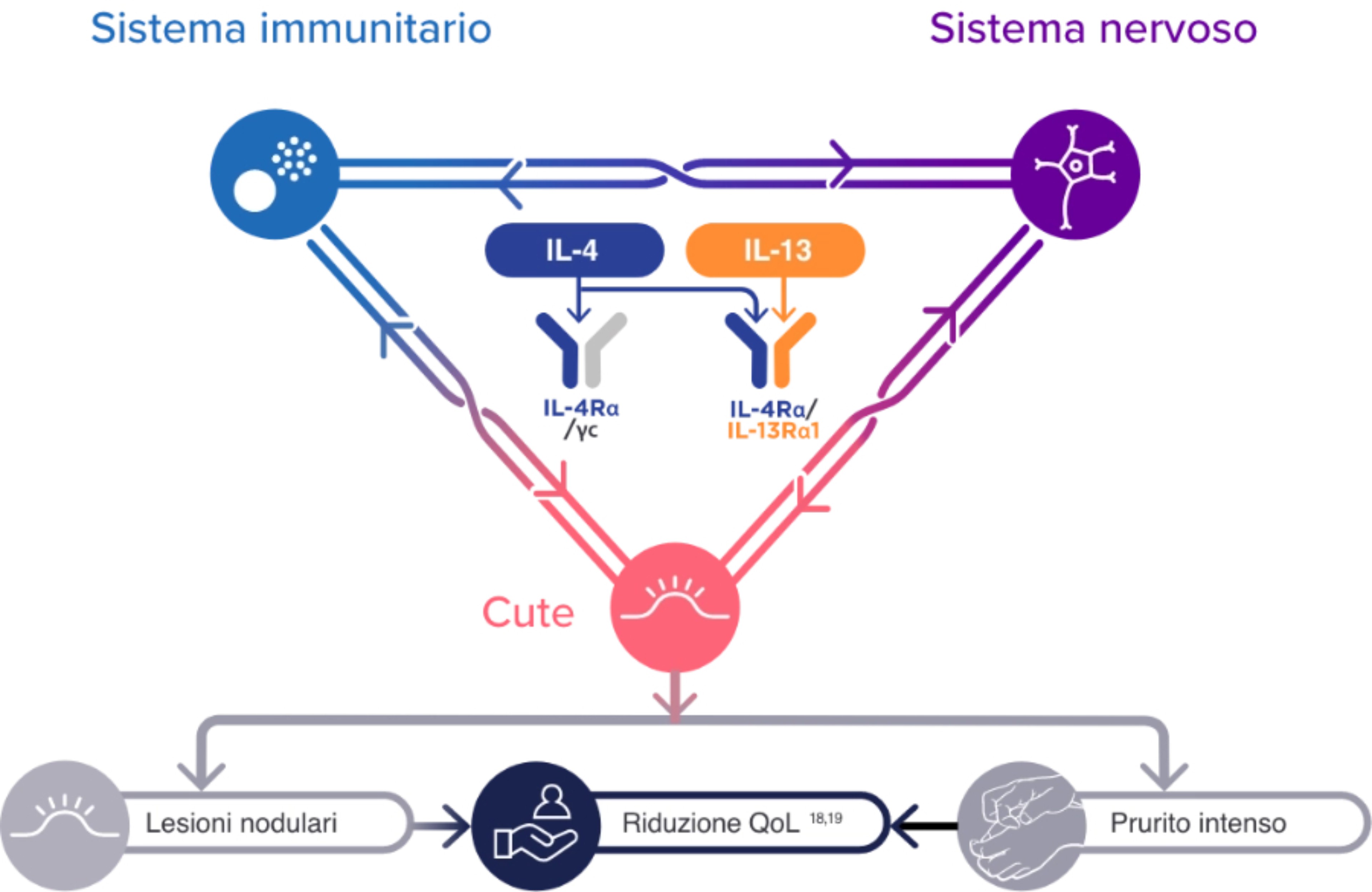
L'aumento dell'attività delle cellule immunitarie di tipo 2 e delle citochine, insieme ad altre cellule immunitarie e mediatori infiammatori, può contribuire ai segni e ai sintomi della PN tramite le interazioni con i neuroni e le cellule dermiche ed epidermiche residenti1-10
Bibliografia
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37(5):578-586.
- Williams KA, Huang AH, Belzberg M, Kwatra SG. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83(6):1567-1575.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14(1):67-77.
- Fukushi S, Yamasaki K, Aiba S. Nuclear localization of activated STAT6 and STAT3 in epidermis of prurigo nodularis. Br J Dermatol. 2011;165(5):990-996.
- Park K, Mori T, Nakamura M, Tokura Y. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21(1): 135-136.
- Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy. 2011;66(8):1107-1113.
- Nakashima C, Ishida Y, Kitoh A, Otsuka A, Kabashima K. Interaction of peripheral nerves and mast cells, eosinophils, and basophils in the development of pruritus. Exp Dermatol. 2019;28(12):1405-1411.
- Hashimoto T, Rosen JD, Sanders KM, Yosipovitch G. Possible roles of basophils in chronic itch. Exp Dermatol. 2019;28(12):1373-1379.
- Sonkoly E, Muller A, Lauerma AI, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411-417.
- Fostini AC, Girolomoni G, Tessari G. Prurigo nodularis: an update on etiopathogenesis and therapy. J Dermatolog Treat. 2013;24(6):458-462.
- Mack MR, Kim BS. The itch-scratch cycle: a neuroimmune perspective. Trends Immunol. 2018;39(12):980-991.
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36(3):189-197.
- Kwatra SG. Breaking the itch-scratch cycle in prurigo nodularis. N Engl J Med. 2020;382(8):757-758.
- Janmohamed SR, Gwillim EC, Yousaf M, Patel KR, Silverberg JI. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313(8):669-677.
- Pereira MP, Hoffmann V, Weisshaar E, et al. Chronic nodular prurigo: clinical profile and burden. A European cross-sectional study. J Eur Acad Dermatol Venereol. 2020;34(10):2373-2383.
- Campion M, Smith L, Gatault S, Métais C, Buddenkotte J, Seinhoff M. Interleukin-4 and interleukin-13 evoke scratching behaviour in mice. Exp Dermatol. 2019;28(12):1501-1504.
- Edukulla R, Singh B, Jegga AG, Sontake V, Dillon S, Madala S. Th2 cytokines augment IL-31/IL-31RA interactions via STAT6-dependent IL-31RA expression. J Biol Chem. 2015;290(21):13510-13520.
- Nguyen JK, Austin E, Huang A, Mamalis A, Jagdeo J. The IL-4/IL-13 axis in skin fibrosis and scarring: mechanistic concepts and therapeutic targets. Arch Dermatol Res. 2020;312(2):81-92.
- Serezani APM, Bozdogan G, Sehra S, et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J Allergy Clin Immunol. 2017;139(1):142-151.e5.
MAT-IT-2302970